She can precisely create a force three times greater than the geocentric pressure by precisely screwing a few screws on top of a small metal cone. Surprisingly, she and her colleagues at Bayreuth University have discovered a superhard material that can withstand this tremendous pressure. Its hardness is so high that it can leave dents on the diamond. Diamond has long been considered the hardest substance in the world.
This new substance is an achievement of decades of modern alchemy efforts, and scientists have been modifying the chemical structure of matter to change its properties. Many of them chose the wrong starting point, and many people walked into the dead end, but these recent achievements may have a wide-ranging impact, from breakthroughs in medical technology to changing our understanding of the distant world.
Human love for hard materials dates back to ancient times. Our ancestors used hard stones to grind other softer stones into blades. Later, these stones were gradually replaced by harder materials until 2000 when humans invented steel. Steel was once the hardest material known, until the end of the 18th century scientists discovered that they could coat the surface of the tool with a layer of diamond.
Although diamond can be made into jewellery, most diamonds are made into a super-hard coating after processing, wrapping the tool and the drill bit to reduce wear. In the mining and petroleum industries, diamond drill bits are indispensable – without it, they cannot dig into the underground hundreds of meters to gain valuable resources. “Ultra-hard coatings are used in a variety of applications, from high-speed cutting machines to deep-sea drilling, to natural gas and oil exploration, to biomedical applications,†said Jagdish Nara, professor of materials science at North Carolina State University. Yang (Jagdish Narayan) said.
To understand the reason why a material is hard, it is necessary to study the atomic structure of its crystal.
The carbon atoms constituting the diamond are also components of soft graphite, and the graphite can be made into a pencil lead. The difference between the two carbon materials is the difference in the arrangement of the atoms. In graphite, carbon atoms are arranged in a planar hexagonal layer with weak chemical bonds between each layer.
In diamonds, carbon atoms are tightly connected in the form of tetrahedrons, which are very strong. Together with the strong chemical bonds between the carbon atoms, the diamond becomes very hard.
The word "diamond" itself comes from the ancient Greek adámas, meaning indestructible. However, when the pressure reaches a certain size, the diamond will also break, and the small flaws in the crystal structure will also make it weak, making the diamond easy to disintegrate.
For scientists, this can be problematic – what if you need to use high pressures while studying the characteristics of the material, and the hardest materials naturally produced on Earth cannot withstand this pressure? You need to find something harder.

It is no wonder that the study of superhard materials began with an attempt to replicate the structure of the diamond, but only a few elements could form the chemical bonds of the diamond.
One of them is boron nitride. Like carbon, it is polymorphic, and nitrogen and boron atoms can form a diamond-like structure instead of carbon atoms. In 1957, scientists made a boron nitride cube, which was initially said to cause scratches on diamonds, but soon the dream became a bubble, and tests showed that it was less than half the hardness of diamond.
Over the next few decades, scientists established chemical bonds between these three elements—nitrogen, boron, and carbon—in many ways, but none of them succeeded. In 1972, scientists finally used this material to make a film that was similar in structure to diamond, but the disadvantage was that it required complex chemical reactions and high temperatures. Until 2001, researchers at the National Academy of Sciences of Ukraine in Kiev worked with their colleagues in France and Germany to produce diamond-like boron-carbon nitrogen. Although the hardness of this new material exceeds that of boron nitride cubes, it is still slightly inferior to diamond.
Seven years ago, Chen Changfeng, a physicist at the University of Nevada, and his colleagues at Shanghai Jiaotong University thought they found something harder than diamonds. According to their calculations, a strange hexagonal boron nitride (also known as wurtzite boron nitride) has a hardness of more than 18% of diamond. This rare material has a structure similar to that of a diamond tetrahedron or a boron nitride cube, which differs in the angle of chemical bonds. Computer simulations have shown that these chemical bonds are flexible under high pressure and are adjusted to a 90 degree angle to relieve pressure.
Although the chemical bonds of diamonds have a similar response when subjected to pressure, the hardness of wurtzite-type boron nitride is increased by 80% under high pressure. Surprisingly, the manufacturing process of wurtzite-type boron nitride is quite dangerous – it will only be produced in the high temperature and high pressure environment of natural volcanic eruptions, and artificial synthesis must mimic the explosive environment. It is very difficult to obtain a sufficient amount of wurtzite type boron nitride, and this remains to be tested. The study of another related substance, lonsdaleite, has also encountered a similar dilemma.
This material can withstand a pressure of more than 58% of diamond.
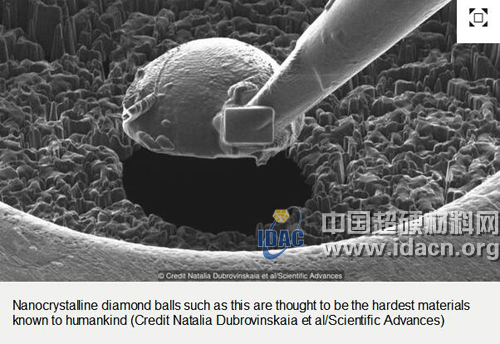
This nanocrystalline diamond sphere is considered to be the hardest material available today.
The structure of this material consists primarily of diamond-like chemical bonds and 10% to 15% of graphite-like chemical bonds. The research team's tests showed that Q-carbon is at least 60% harder than diamonds, but it has not yet been finalized. A true hardness test requires drilling a hole in it with a harder material than it is. When the Q-carbon sample was extruded using two diamond drill bits, the drill bit was deformed. Narayan said.
At this time, the Dubrovin Winskea anvil appeared on the scene. Her new material is a unique carbon phase called the Nanocrystalline Diamond Ball. It is not a single crystal structure like the one used for the engagement diamond ring, but a large number of small crystal structures - each crystal is only one-tenth the size of the hairline - they are connected by a layered structure of graphite. The wonderful material of this Nobel Prize level is only one carbon atom thick.
Diamonds will shatter at a pressure of 120 billion kPa, and this new material can withstand at least 460 billion kPa. When multiple layers of new materials are pressed together to produce a high pressure of 100 billion kPa, it will not break. This small sphere is already the hardest thing known on earth. For example, this is equivalent to 3,000 adult African elephants standing on the stiletto of a high heel. “This is the hardest of all known superhard materials, and it can be used to extrude traces on all other materials,†says Dubrovin.
These nanocrystalline diamond spheres are still transparent, and researchers can use them as tiny lenses to observe the extruded material with X-rays. “We can use it to see what happens to the extruded material.†Dubrovinskea said, “Ultra-high pressure opens up new horizons for deeper understanding of matter.â€
Dubrovinsky and her have also studied cockroaches with this material.锇 is a kind of metal with strong resistance to pressure. They found that 锇 can withstand more than 750 billion kPa of squeezing force. When this pressure is reached, the electrons and protons that are originally connected to each other within the metal atom begin to interact. Researchers believe that this stranger phenomenon may cause the metal to change from a solid to an unknown state of matter. They hope that in the future they will be able to study what attributes this will bring to them.
These superhard nanodiamonds have surpassed the ability to provide new hard edges to cut stones and metals. Powdered nanodiamonds can be used in the cosmetic industry, which is highly absorbent and can be combined with active materials. They are easily absorbed by the skin, which brings the active substance into the skin. The pharmaceutical industry is also beginning to study its use, for example, it can bring drugs into parts of the human body that are difficult to reach with chemotherapy. Studies have also shown that nanodiamonds can promote the growth of bone and cartilage.

Schematic diagram of the broken sample in the diamond pressure chamber
Under such pressure, the elements will change strangely. Nitrogen, for example, a normal gas on Earth, becomes like a metal and begins to be electrically conductive. Dubrovins and Dubrovinsky hope that their superhard diamonds will help us create these environments in the universe. “We can start to simulate the inner environment of a giant planet or the super-earth of the solar system.†Dubrovin Keskea said, “What I find even more amazing is that the equipment that allows us to do this is so small that it can be held in both hands. live."
(The author is By Richard Gray; the article was published on the BBC website on August 16, 2016. The main content of the article is translated by "British Network", and some of the content is translated by China Superhard Materials.)
Original address: Click to read
I System description:
Heat Shrinkable Sleeve with hot melt adhesive, having properties of excellent insulating, environmental sealing, and resistant to impact and abrasion. It is designed for applications to seal and protect electrical splices, cable terminations and joints where electrical insulation and water proof are required. 3:1 shrink ratio allows it easily fit over irregular shape and large connectors.
II Features
>Resistant to UV-radiation
>High electrical insulation
>Superior mechanical property
>Minimum fully recovery temperature: 120°C
>Standard color: Black
III Techinal Data
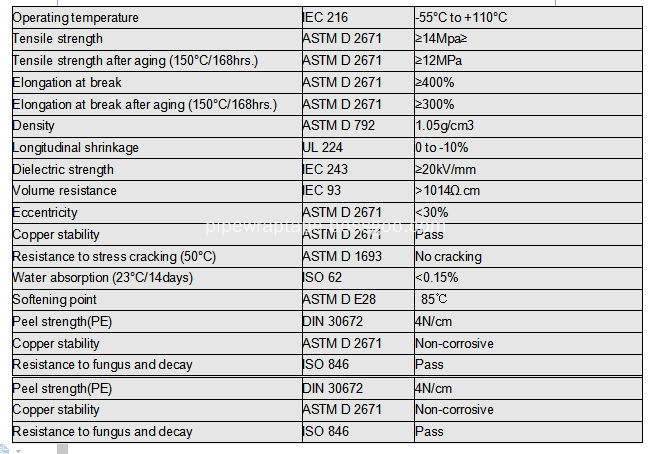


Heat Shrinkable Sleeve
Heat Shrinkable Sleeve, Pipe Heat Shrinkable Sleeve, Pvc Heat Shrink Sleeve, Hot Shrink Sleeve, Corrosion Protection Tape
Jining Xunda Pipe Coating Materials Co.,Ltd , http://www.pipe-wrap.com