Adding additional solvents in the production of plastic solar cells can increase their conversion efficiency by 2 or 3 times. To address this issue, researchers at the University of Technology in Eindhoven, Netherlands (TU/e) have now found the answer.
The extra solvent plays a role just like baking powder mixed in the dough. Over the past decade, the principle of how solvents are added has been unclear, and today TU/e ​​researchers explained this mechanism in a recent issue of Nature Communications and developed plastic solar cells. Opens a new direction.
Plastic solar cells or organic solar cells use polymers to replace the commonly used twins and convert energy into electrical energy. Using plastics as a basic material reduces the cost and weight of these solar cells and makes them flexible. However, the resulting battery efficiency is about 10%, still lower than the efficiency of about 15 to 20% that can be achieved with commercial twin-crystal solar cells.
About ten years ago, researchers stumbled upon the addition of additional solvents (cosolvents) in the production of plastic solar cells that could increase their conversion efficiency by two to three times. Prof. TU/e ​​Professor Ren Janssen explained that these co-solvents are now used in a variety of plastic solar cells, but no one really knows why it can bring such a good conversion efficiency.
It is understood that this is related to the shape of the solar cell - that is, the actual structure of the two hybrid plastic components between the electrons in the cell that move with the sunlight. The composition of both organic materials dissolves during the production process and then evaporates and hardens. This mysterious co-solvent must usually be added to the solvent before evaporation.
The TU/e ​​researchers led by Ren Janssen used an optical technology combination to find the exact answer. If no co-solvent is added, larger droplets will form during the hardening of the plastic mixture. These droplets are not conducive to electron transport, thereby affecting the efficiency of solar cells. The more co-solvent is added to the solution, the smaller the bubbles formed until disappearing completely.
Researchers also discovered its causes. Two effects occur in the hardening process, Janssen explained: First, the solution evaporates, and the polymer exhibits a folded structure. We see that the co-solvent can begin this folding process at an earlier stage, which means that bubbles will no longer form. The co-solvent acts like a baking powder in this way, improving the structure of the mixture and helping to increase the efficiency of the solar cell.
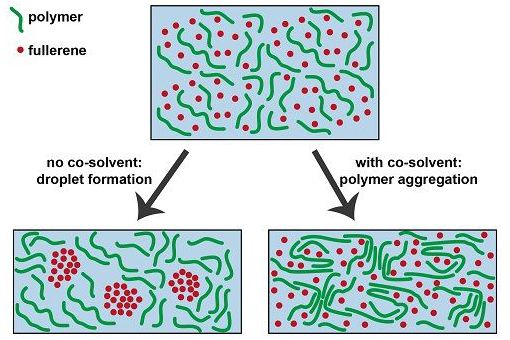
The figure shows the effect of co-solvents on the solution of two plastic components (polymers and fullerene molecules) during the production of plastic solar cells. The fullerene molecule with co-solvent droplets limits the efficiency of the solar cell; the co-solvent-added polymer molecules form the folded structure faster, avoiding bubble formation.
Binder Course,Binder Layer,Bituminous Binder Course,Underlying Layer Environmental Adhesive
Zibo Century Union New Building Materials Co.,Ltd , https://www.cum-sports.com