Abstract The University of Tokyo announced on August 25, 2016 that it was found that a single component can achieve the coexistence of more than four phases under special conditions. This achievement not only helps to make a basic understanding of the phase coexistence in the state of thermal equilibrium, but also has the ability to utilize small thermodynamic disturbances...
The University of Tokyo announced on August 25, 2016 that it was found that a single component can achieve the coexistence of more than four phases under special conditions. This achievement not only contributes to the basic understanding of the phase coexistence in the state of thermal equilibrium, but also has the characteristics of using small thermodynamic perturbations to control the phase transition between phases, and therefore is expected to utilize functional materials of phase change. Contribution to development. 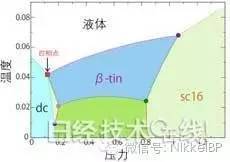
Temperature-pressure phase diagram in the parameters of the four-phase point of the Stillinger-Weber model
The dot indicates the triple point, and the square indicates the four-phase point (picture is from the press release of the University of Tokyo)
In terms of multiphase coexistence, everyone knows that water and ice coexist in equilibrium at 0 °C. In addition, the three-phase point where gas, liquid, and solid three-phase coexist in the water molecule exists in the high temperature and high pressure range. A condition in which a plurality of thermodynamic phase states coexist in an equilibrium state can be imparted under conditions in which chemical potential energy is equal. Among the single-component substances, the chemical potential energy is only a function of the thermodynamic variables such as temperature and pressure. According to this fact, it is concluded that more than three phase states generally cannot coexist in the thermal equilibrium state. This is the famous "Jibu". Phase of the law." However, considering it as a mathematical problem, as a special solution, there may be cases where four phases coexist, and no one has systematically studied the existence possibility of four-phase points.
This time, the researchers at Dongda conceived a method to systematically change the interaction between atoms by using a certain variable, to give the original system a virtual degree of freedom, and to improve the dimension of the parameter space, thereby systematically Look for the four-phase point. The isotropic two-body interaction part of the Stillinger-Weber potential energy used to describe the movement of silicon atoms, and the anisotropic three-body interaction part used to orient the independent tetrahedral structure, by comparing the ratio of the two As a new variable introduction system, the phase diagrams of the temperature, pressure and new variables in the three-dimensional parameter space were created.
Through the above method, the researchers discovered the three-phase coexistence line as the intersection line of the two-phase coexistence surface, and finally found the intersection point of the three-phase coexistence line, that is, the four-phase point. By changing the potential energy variable, the movement of atoms such as silicon, helium, water, carbon, etc. can be described. The researchers conclude that the newly discovered variable value is located near the 锗. The four phases coexisting are liquid, β-tin crystal, diamond cubic (dc) and SC16 crystal.
In the vicinity of this four-phase point, subtle changes induce phase transitions, and in the phase transition between solids, mechanical elastic strain sometimes hinders phase transition. However, there is a possibility that the elastic phase strain is reduced under the multiphase coexistence phase state combination, showing the usefulness in application. In fact, researchers have recently discovered advanced functions of the highly related substance VO2 that are expected to be applied to optical switching elements near the triple point.
It is not easy to control the interaction, but it is expected to occur even in the actual system by using the recently developed colloids with patches (different areas of physical properties) and relatively easy-to-control interactions using electrostatic interactions. Exceeding the multi-phase coexistence possibility of Gibbs's law. In addition, even when it is difficult to reach the four-phase point, there is a possibility of more flexible phase control in the vicinity thereof, and therefore, the result of this time is expected to have a great impact on application. (Contributing author: Kudo Kudo)
Kylin Chemicals manufactures & supplies high performance Acrylic dispersant polymers & thickeners, serves a variety of use in the following applications.
Detergents and Laundry
- Acrylic acid - Maleic acid copolymer, counterparts for BASF Sokalan CP45, Sokalan CP5, Sokalan CP7, Acusol 497N
- Acrylic acid homopolymer, counterparts for BASF Sokalan PA 25 CL, DOW Acusol 445, Acusol 445N

Cosmetics and Personal Care
- Cross-linked polyacrylate polymer, counterparts for Carbomer 934, Carbomer 940, Carbomer 941, Carbomer 980, Carbomer 981, Carbomer 1342, Carbomer 1382 and Carbomer 2020, etc.

Our advanced DCS manufacturing systems, advanced analytical instruments and quality systems assure the quality, stability and sustainability to your supply chain needs.
Acrylic Dispersant Polymers & Thickeners
Acrylic Dispersant Polymers,Acrylic Dispersant Thickeners,Ethylene Diamine Tetraacetic Acid,Kl Carbomer
Kylin Chemicals Co., Ltd. , http://www.kylin-chemicals.com